Combines Extensive Contact Lens & Pharma Experience
Technology
Technology Background
A Targeted, Tailored and Sustained Drug Treatment

Targeted
Significantly Increases bioavailability by 10-20x as compared to eye drops

Tailored
Ability to deliver multiple drugs to the eye via the Contact Lens

Drug Range
Both hydrophobic and hydrophilic with a molecular weight of 70 – 1500 & concentration of 20 to 50 mg/ml
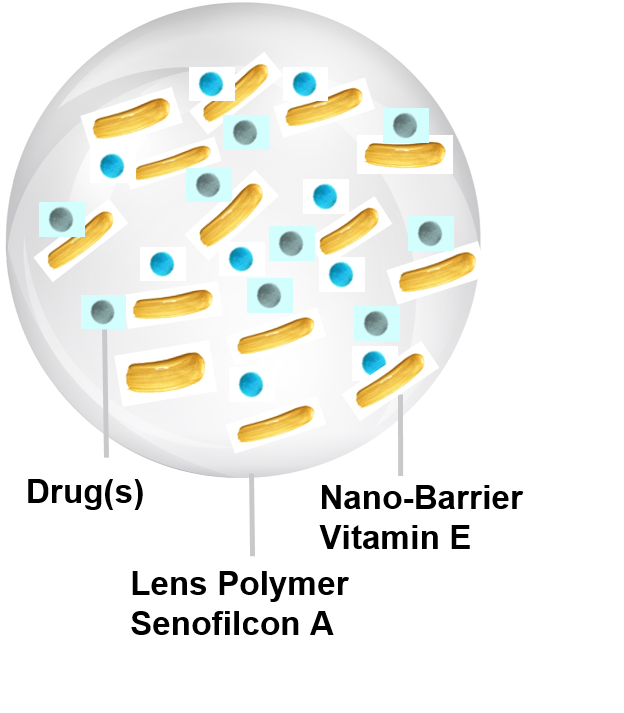

Sustained
Delivers drugs consistently for extended durations - no need for multiple daily eye drop instillations. Release rates increased and decreased by Vitamin E concentration

Commercial and Safe Raw Materials
Approved Bandage Contact Lens (Senofilcon A), USP Drugs, USP Vitamin E, Preservative-Free, 0.5% Polyvinylpyrrolidone
Freya Technology Platform
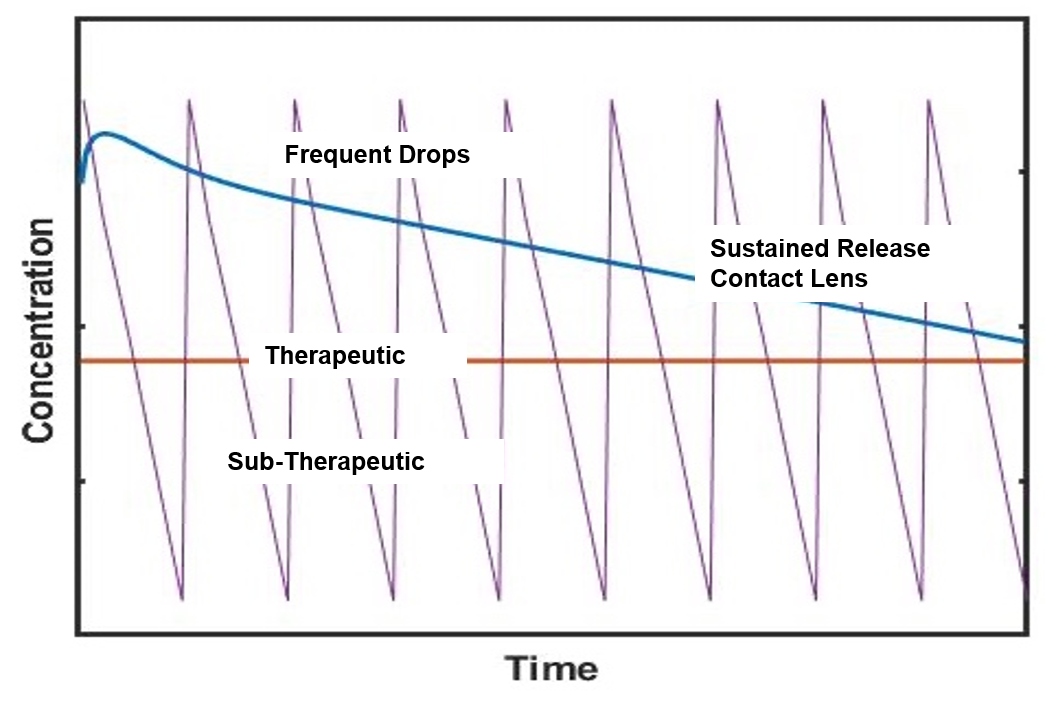
Providing Significant Improvements over Yesterday’s Eye Drop Technology
Provides Unique
Sustained Release
Continuous
Therapeutic Dose
Removes Compliance
& Instillation Issues
Technology Applications
$1.5B Markets
FDA Approved Senofilcon A Contact Lens


FDA Approved Drugs


Indication
