Combines Extensive Contact Lens & Pharma Experience
Moxi-Lens™
Moxi-Lens™
For Post Ocular Surgery Freya has developed and demonstrated MoxiLensTM - antibiotic eluting contact lenses provide sustained release resulting in therapeutic concentrations for extended durations, eliminating the peaks and troughs in concentrations from drops, which may cause sub-therapeutic concentration between drop instillations, which can lead to infection proliferation and/or development of resistance
Worldwide
0
Milion +
US
0
Milion +
Bacterial Conjunctivitis Infections per Year

Moxi-LensTM: In VITRO DATA
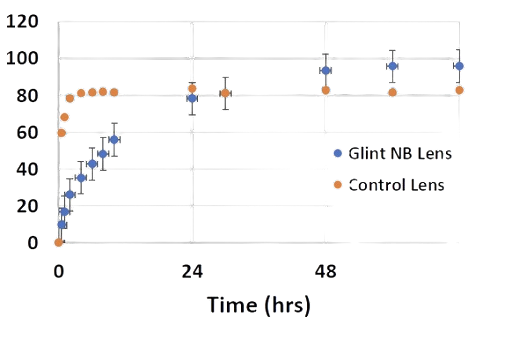
MoxiLens™ releases drug for 72 hours - vs just 2 hours for a conventional lens
Lab Tests indicate a 72-hour antibiotic treatment for the Moxi-LensTM vs. non-vitamin E control lens
Mass Release (µg)
Lab Tests indicate a 72-hour antibiotic treatment for the Moxi-LensTM vs. non-vitamin E control lens
Physician Usage
Feedback on Moxi-LensTM has been very positive
Post Surgical
“good results”
Corneal Erosion
“Worked well”. “It is a game changer”.
“MoxiLensTM lens was used successfully over 3 days to treat a recurrent corneal erosion after surgical complications. The lens was comfortable and healed the epithelial defect.”
Post PRK
“Healed without issues. Used for 4 days. Comfortable. Also, prescribed FML drops.”
Corneal Erosion
”Used Moxi-LensTM in patients with pathology (ABMD, RCE, etc) & refractive error. Both patients found lens comfortable & reduced the number of drops & cost was noted. Rehab times were quick with rapid recovery in all patients”
Corneal Abrasion
“I often use a bandage CL to provide comfort & protection for treating large corneal abrasions. The MoxiLensTM added another layer of confidence that the cornea would heal with minimal risk of infection.”
“Used to treat an abrasion for 48 hours and then used drops to finish the job.” “Used on an older patient who had a corneal abrasion & noncompliant with drops. The abrasion healed without incident or discomfort.”
General
“The Moxi-LensTM provided excellent coverage & comfort, promoting healing while delivering therapeutic levels on an antibiotic.” “ The MoxiLensTM is very beneficial addition to my toolbox for treating corneal disease. And provides 24-hour antibiotic delivery to the cornea – improves compliance.”
Corneal Ulcer
“The patient was not a previous CL wearer. The patient had no discomfort whatsoever. The patient was seen daily for the first 2-3 days, then again 3 days later. The CL stayed in the eye for 7 days with no additional topical meds for one week. No problems. I would definitely store MoxiLensTM on the shelf in case of an emergency.”
FDA Approval Target Product Profile
Vitamin E modified bandage contact lens loaded with Moxifloxacin hydrochloride ophthalmic solution for treatment of bacterial conjunctivitis (Moxi-LensTM)
Ingredients
A senofilcon A contact bandage lens

1.5%
moxifloxacin
Hydrochloride
20%
vitamin E
0.5%
polyvinylpyrrolidone
Dosage
1 Moxi-LensTM worn in infected eye continuously up to 72 hours
Dosage

Single use vials with > 1 year shelf life
Target Duration

72 hours
Reimbursement

Reimbursement when FDA approved
Target Endpoints
Moxi-LensTM is a disposable senofilcon A bandage drug-eluting contact lens packaged with a topical fluoroquinolone anti-infective indicated for the treatment of bacterial conjunctivitis These lenses have been approved for daily and extended wear for up to 3 or 6 nights/4 or 7 days of continuous wear. Pediatrics (12 years of age and older): The safety and efficacy of the MoxiLensTM has been established.
Clinical Plan
Clinical Development Plan Rapidly Progressing Toward 2026 MoxiLens™ NDA Filing

2H 2022
- SBIR Grant Received
- IND Accepted
- Safety Study Completed

3Q 2023
- FDA Type C Meeting held September 6 providing program agreement per IND update
- Select & Prepare CRO
- Phase 2 Clinical Begins
2025
- Phase 2 Clinical Complete
- Phase 2 Data Review
- Select & Prepare CMO

1H 2026
- Initiate Phase 3 Trial
